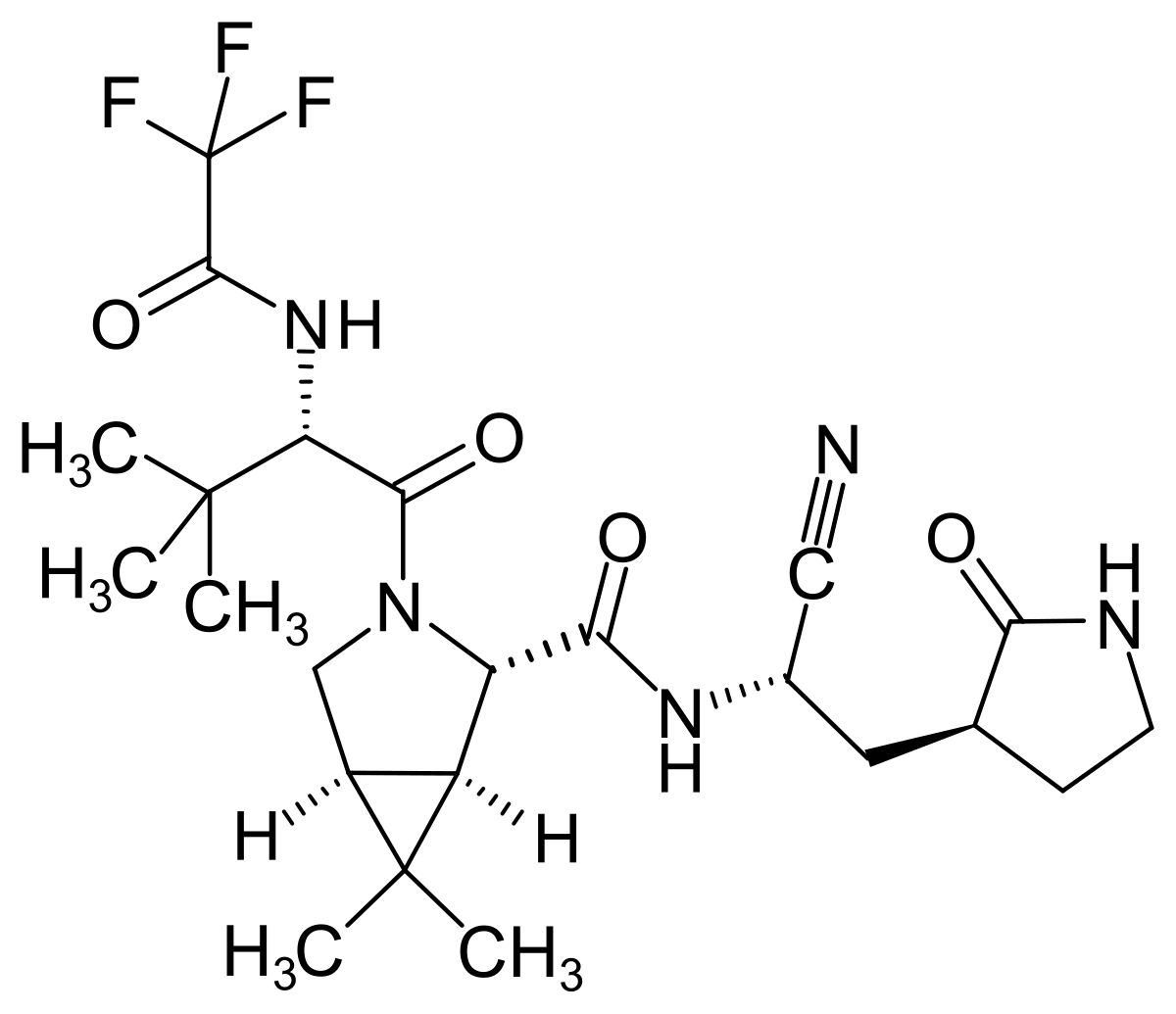
Paxlovid Structure | Mzcwdxudzfa7km
A pill to treat Covid Pfizer and Merck both generated excitement with news they have pills that could help keep people out of the hospital if they catch coronavirus. Nucleophilic attack by the main proteases Cysteine 145 Residue leads to a covalent bond structure on the right.
23 hours agoPaxlovid also known as PF-07321332 is an oral pill used to treat COVID patients as soon as they are aware they have been exposed to the virus or at the first sign of infection.
Paxlovid structure. Patient receiving Paxlovid also did better in the those treatment-emergent adverse events with fewer serious adverse events 17 vs. 1 day agoThe statement from Pfizer comes at a time when the researchers across the globe are racing to find a pill against COVID-19 that can be taken at home to. Patients take two packs a day for five days.
Pfizer has reported a successful clinical trial of its experimental drug Paxlovid which has been studied in the treatment of adult patients with coronavirus infection COVID-19 caused by the new coronavirus SARS-CoV-2. The medicine comes in a blister with two Paxlovid pills and one of an antiviral ritonavir which allows Paxlovid to stay active longer at higher concentrations. Pfizer says newly developed antiviral pill stops hospitalization and death by 89.
Much like the anti-HIV drugs completely changed the course of the terrifying AIDS epidemic. In the trial just six of more than 600 volunteers were hospitalized after taking Paxlovid. New York NY Pfizer Inc.
PFE today announced its investigational novel COVID-19 oral antiviral candidatePAXLOVID significantly reduced hospitalization and death based on an interim analysis of the Phase 23 EPIC-HR E valuation of P rotease I nhibition for C OVID-19 in H igh-R isk Patients randomized double-blind study of non-hospitalized adult patients with COVID-19 who. In my opinion this means the adverse effects from COVID were worse than those. Paxlovid a protease inhibitor is the same type of drug that turns HIV into a controlled disease it blocks the replication of the virus.
PAXLOVID PF-07321332 is a protease inhibitor that has demonstrated potent in vitro antiviral activity against SARS-CoV-2 and activity against other coronaviruses suggesting potential for use in the treatment of. The drug comes in a blister pack with two pills of. By the end of the year the company plans to complete two other studies of the pill which is given.
18 hours agoAccording to Pfizer. The drug - Paxlovid - is intended for use soon after symptoms develop. The Pfizer drug Paxlovid achieved an 89 reduction in risk of hospitalization or death Pfizer CEO Albert Bourla said.
Paxlovid is a protease inhibitor the same type of drug that turned HIV into a manageable disease which blocks replication of the virus. The COVID era is reteaching us a lesson that was learned in the 1990s and 2010s that an effective direct-acting antiviral DAA medication can work wonders in controlling or eliminating a serious viral infection. The drug comes in a blister pack with two pills of Paxlovid and one of an antiviral ritonavir which enables Paxlovid to remain active longer at higher concentrations.
Pfizers PAXLOVID PF-07321332 is an oral antiviral therapeutic targeting the SARS-CoV-2 betacoronavirus to prevent COVID-19. Pfizers Paxlovid is the second anti-Covid-19 pill after that of Merck which is actually an influenza medicine rebranded to fight the coronavirus. The mechanism of action of PAXLOVID is mainly derived from its nitrile -CN group.
PFE today announced its investigational novel COVID-19 oral antiviral candidatePAXLOVID significantly reduced hospitalization and death based on an interim analysis of the Phase 23 EPIC-HR Evaluation of Protease Inhibition for COVID-19 in High-Risk Patients randomized double-blind study of non-hospitalized adult patients with COVID-19 who are at. 19 hours agoThe chemical structure of Paxlovid. The drug Paxlovid is intended for use soon after symptoms develop in people at.
A new drug being developed by Pfizer offers the possibility of nearly putting an end to deaths from COVID-19. 1 day agoCovid-19 pill. They noted that patients bailed from the trial more often in the placebo group 41 rather than the paxlovid group 21.
The outcomes in the middle-to-late stage medical trial were so strong that Pfizer stop recruiting new people for that trial it stated. Although this is a covalent bond the structure formed. PAXLOVID is an investigational SARS-CoV-2 protease inhibitor antiviral therapy combining PF-07321332 and ritonavir.
Pfizer has announced a pill it is testing called PAXLOVID dramatically reduces COVID-19 deaths and hospitalizations in patients. PAXLOVID was found to reduce the risk of hospitalization or death by 89 compared to placebo in non-hospitalized high-risk adults with COVID-19 In the overall study. An experimental pill to treat Covid developed by the US company Pfizer cuts the risk of hospitalisation or death by 89 in vulnerable adults clinical trial results suggest.
The drug is available in blister packs consisting of two Paxlovid tablets and antiviral ritonavir which allows Paxlovid to remain active at high concentrations for a longer period of time. All studies Mortality Hospitalization Serious outcomes RCTs RCT mortality All outcomes 0 025 05 075 1 125 15 175 2 All studies 95 1 1219 Improvement Studies. The Pfizer drug called Paxlovid achieved an 89 percent decrease in chance of hospitalization or dying among adult patients with COVID whore at high-risk of progressing to certain illness the united states company stated.
Paxlovid is a protease inhibitor the same type of drug that turned HIV into a manageable disease which blocks replication of the virus. Paxlovid is a protease inhibitor the same type of drug that made HIV a manageable disease blocking the replication of the virus. An experimental pill to treat Covid developed by pharmaceutical giants Pfizer cuts the risk of hospitalisation or death by 89 in vulnerable adults the company has announced.
PAXLOVID Oral Antiviral PF-07321332 Description. Pfizer says its pill which it proposes to sell under the brand name Paxlovid if it gets authorized reduced the risk. Among the 1219 people included in the early analysis no one who.
1 day agoPfizer said its double-blind study examined a group of non-hospitalized high-risk adults with COVID-19. 5Scientists at Pfizer created Paxlovid with the help of the ultrabright X-rays of the Advanced Photon Source. New York NY Pfizer Inc.
16 hours agoPharmaceutical company Pfizer has announced the results of clinical trials of its new oral antiviral treatment against COVID-19The new drug candidate Paxlovid proved to be effective against the SARS-CoV-2 virus which causes COVID-19 according to results released by Pfizer on Nov. Administration of Paxlovid to patients with mild-to-moderate COVID-19 who were at risk of severe disease resulted in an 89. When given within five days of the onset of symptoms the antiviral therapy called Paxlovid prevented almost 90 of deaths from COVID-19 compared to a placebo a Pfizer study found.
Pfizer said its medication had been created specifically to fight Covid-19.

Pfizer Says Its Antiviral Pill Paxlovid Cuts Hospitalisation Rate Death Risk Due To Covid By 90
The Era Of Anti Covid Pills Begins

Experimental Pfizer Pill Prevents Covid Hospitalizations And Deaths Stat

Zeitschrift Fur Infektionstherapie Die Zeitschrift Infektio Aktuell














/i.s3.glbimg.com/v1/AUTH_63b422c2caee4269b8b34177e8876b93/internal_photos/bs/2021/E/2/5UB8lqR7K5r3aaZSj2hw/ap21035598811864.jpg)
